BY


From reading this blog, you may know that nanomaterials are becoming more and more prevalent in the products that we use on a regular basis. What you may not realize is that nanomaterials are also becoming a growing part of our food packaging. In this post, I’m going to explore a few ways in which nanomaterials might be used in food packaging to improve packaging properties and to more accurately determine a product’s shelf life.
Biodegradable packaging helps reduce the waste in our landfills and elsewhere, but biodegradable plastics have been a challenging choice for food packaging in the past for several reasons. Experts have written that these materials aren’t as strong or heat resistant as other, more commonly used plastics, air and water can get through them more easily, and they can be difficult to work with.1 On the bright side, there are nanomaterials being developed for use in plastics for food packaging that can help get around some of these problems. Scientists are working hard to develop these nanocomposites to be biodegradable and still usable in food packaging.2

Creating biodegradable plastics for food packaging can be tricky. image source
While on the topic of packaging, let’s take a look at another major concern for the food industry: food spoilage.3 Each year, the US throws away 40% of our food supply,4 and a portion of this waste is due to food spoilage. Food spoils because of unwanted microbial growth, and one way to control this growth is to remove a key component in microbe survival – oxygen! You might think that being “air tight” is part of the point of having packaging in the first place, but consumers like to see the food that they are buying, and unfortunately most transparent packaging is not very good at keeping out oxygen. This is where the nanomaterials come in. Nanoparticulate clay material can be added to polymer packaging material to keep the packaging transparent while also significantly reducing oxygen diffusion.5-7 Limiting oxygen content inside food packaging is an important part of reducing unwanted microbial growth in our foods.8
In addition to limiting oxygen diffusion through the packaging barrier, nanomaterials can also help prevent food spoilage by scavenging oxygen that has made it through the packaging barrier. Think about the small silica gel desiccant packets that you may have seen packaged with various products such as shoes, handbags, and electronics. When placed inside the packaging of those products, the silica gel packets can adsorb up to 40% of their weight in water out of the air, keeping the items boxed up with them dry.9 Oxygen scavenging nanomaterials can be thought of operating in a similar way, but are much, much smaller than the silica spheres, and they react with oxygen molecules instead of adsorbing water.

Silica gel adsorbs water from the air. image source
One example of an oxygen scavenger is ascorbic acid, better known as vitamin C. For those interested in the specific chemistry, the reaction is as follows:10
2C6H8O6 + O2 = 2C6H6O6 + 2H2O
Remember our explanation about moles a couple posts ago? This reaction indicates that 2 moles of ascorbic acid (C6H8O6) will react with 1 mole of oxygen to produce 2 moles of dehydroascorbic acid (C6H6O6) and 2 moles of water. In other words, vitamin C and oxygen go in and dehydroascorbic acid and water come out. No more oxygen. Vitamin C has more uses than just keeping colds away! The difficulty with this reaction is that, without the addition of a catalyst, it happens quite slowly.10 A catalyst is a material that speeds up a reaction without being depleted itself in the process. In this particular reaction, copper is often used as the catalyst. Ascorbic acid isn’t a nanoparticle, but scientists at Clemson University have used a comparable reaction to design nanoparticles that contain similar materials to react with oxygen.11 One benefit of this newly developed nanomaterial is that it does not require a catalyst to increase the rate of reaction with oxygen. No catalyst means less copper use and lower material costs, which is good for industrial applications and for the environment.
Another important source of wasted food in the U.S. is all those items that get thrown away because they have passed their expiration date. But expiration dates are meant to err on the side of caution; a lot of “expired” food might actually be safe to eat.
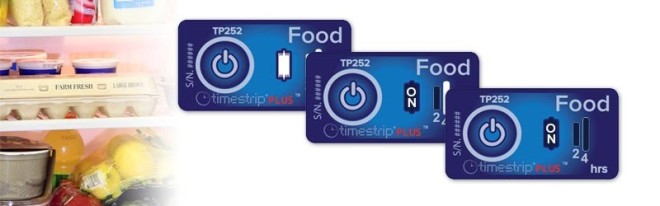
Nanomaterials may be useful for measuring microbial growth in general, or even for indicating the presence of specific types of microbes.3 Nanomaterials could also alert us to changes in temperature for foods or medications that need to remain within a specified temperature range to be safe.3 This kind of nanomaterial time-temperature indicator is already commercially available, and often indicates a change in conditions by changing colors.12 For example, Timestrip® has created the iStrip, which is a packaging indicator that starts out red and changes color if the temperature drops below freezing.12 The technology uses gold nanoparticles, which irreversibly aggregate together at temperatures below freezing.12 The gold nanoparticles change color based on the size of their aggregates, so their temperature-dependent aggregation is very useful for this kind of technology.13

Timestrip’s Cold Chain monitoring labels. image by Timestrip – reprinted with permission
Nanomaterials can help make environmentally friendly food packaging, help prevent or delay food spoilage, and help ensure drug and food safety while minimizing waste. Much research remains to be done to enable scientists to create nano-based breakthroughs like these in the food industry and be assured of their safety. Organizations such as our Center for Sustainable Nanotechnology (funded by the National Science Foundation), the United States National Nanotechnology Initiative, the Project on Emerging Nanotechnologies(funded by the Woodrow Wilson International Center for Scholars in conjunction with thePew Charitable Trusts), the NANOfutures initiative (a European Technology Integrating and Innovation Platform), as well as many other organizations and researchers, are all working to better understand how to harness the versatile properties that make nanotechnology so useful in everyday products.
DISCLOSURE: The CSN, Sustainable-Nano.com, and the author have not received any compensation for writing this post. We have no material connection to Timestrip or the products mentioned.
REFERENCES
1. Liao, M.-L.; Seib, P. A., Chemistry of L-ascorbic acid related to foods. Food Chemistry 1988,30 (4), 289-312. DOI: 10.1016/0308-8146(88)90115-X2. Sorrentino, A.; Gorrasi, G.; Vittoria, V., Potential perspectives of bio-nanocomposites for food packaging applications. Trends in Food Science & Technology 2007, 18 (2), 84-95.DOI: 10.1016/j.tifs.2006.09.004
3. Chaudhry, Q.; Castle, L.; Watkins, R., Nanotechnologies in Food. The Royal Society of CHemistry: Cambridge, UK, 2010; Vol. 14, p 229.4. Council, N. R. D. New Report: America Trashes Forty Percent of Food Supply.http://www.nrdc.org/media/2012/120821.asp (accessed July 2, 2014).5. Duncan, T. V., Applications of nanotechnology in food packaging and food safety: Barrier materials, antimicrobials and sensors. Journal of Colloid and Interface Science 2011, 363 (1), 1-24. DOI: 10.1016/j.jcis.2011.07.017
6. Silvestre, C.; Duraccio, D.; Cimmino, S., Food packaging based on polymer nanomaterials.Progress in Polymer Science 2011, 36 (12), 1766-1782. DOI: 10.1016/j.progpolymsci.2011.02.003
7. Bitinis, N.; Hernandez, M.; Verdejo, R.; Kenny, J. M.; Lopez-Manchado, M. A., Recent Advances in Clay/Polymer Nanocomposites. Advanced Materials 2011, 23 (44), 5229-5236.DOI: 10.1002/adma.201101948
8. Jay, J. M., Food Preservation with Modified Atmospheres. In Modern Food Microbiology, Jay, J., Ed. Springer US: 2000; pp 283-300.9. HowStuffWorks.com What is silica gel and why do I find little packets of it in everything I buy?” http://science.howstuffworks.com/innovation/science-questions/question206.htm.10. Weissberger, A.; LuValle, J. E., Oxidation Processes. XVII. The Autoxidation of Ascorbic Acid in the Presence of Copper. Journal of the American Chemical Society 1944, 66 (5), 700-705.DOI: 10.1021/ja01233a011
11. Byun, Y.; Whiteside, S., Ascorbyl palmitate-β-cyclodextrin inclusion complex as an oxygen scavenging microparticle. Carbohydrate Polymers 2012, 87 (3), 2114-2119.DOI: 10.1016/j.carbpol.2011.10.037
12. Ranjan, S.; Dasgupta, N.; Chakraborty, A.; Melvin Samuel, S.; Ramalingam, C.; Shanker, R.; Kumar, A., Nanoscience and nanotechnologies in food industries: opportunities and research trends. J Nanopart Res 2014, 16 (6), 1-23. DOI: 10.1007/s11051-014-2464-5
13. Kelly, K. L.; Coronado, E.; Zhao, L. L.; Schatz, G. C., The Optical Properties of Metal Nanoparticles: The Influence of Size, Shape, and Dielectric Environment. The Journal of Physical Chemistry B 2002, 107 (3), 668-677. DOI: 10.1021/jp026731y
Fonte: Sustainable Nano1. Liao, M.-L.; Seib, P. A., Chemistry of L-ascorbic acid related to foods. Food Chemistry 1988,30 (4), 289-312. DOI: 10.1016/0308-8146(88)90115-X2. Sorrentino, A.; Gorrasi, G.; Vittoria, V., Potential perspectives of bio-nanocomposites for food packaging applications. Trends in Food Science & Technology 2007, 18 (2), 84-95.DOI: 10.1016/j.tifs.2006.09.004
3. Chaudhry, Q.; Castle, L.; Watkins, R., Nanotechnologies in Food. The Royal Society of CHemistry: Cambridge, UK, 2010; Vol. 14, p 229.4. Council, N. R. D. New Report: America Trashes Forty Percent of Food Supply.http://www.nrdc.org/media/2012/120821.asp (accessed July 2, 2014).5. Duncan, T. V., Applications of nanotechnology in food packaging and food safety: Barrier materials, antimicrobials and sensors. Journal of Colloid and Interface Science 2011, 363 (1), 1-24. DOI: 10.1016/j.jcis.2011.07.017
6. Silvestre, C.; Duraccio, D.; Cimmino, S., Food packaging based on polymer nanomaterials.Progress in Polymer Science 2011, 36 (12), 1766-1782. DOI: 10.1016/j.progpolymsci.2011.02.003
7. Bitinis, N.; Hernandez, M.; Verdejo, R.; Kenny, J. M.; Lopez-Manchado, M. A., Recent Advances in Clay/Polymer Nanocomposites. Advanced Materials 2011, 23 (44), 5229-5236.DOI: 10.1002/adma.201101948
8. Jay, J. M., Food Preservation with Modified Atmospheres. In Modern Food Microbiology, Jay, J., Ed. Springer US: 2000; pp 283-300.9. HowStuffWorks.com What is silica gel and why do I find little packets of it in everything I buy?” http://science.howstuffworks.com/innovation/science-questions/question206.htm.10. Weissberger, A.; LuValle, J. E., Oxidation Processes. XVII. The Autoxidation of Ascorbic Acid in the Presence of Copper. Journal of the American Chemical Society 1944, 66 (5), 700-705.DOI: 10.1021/ja01233a011
11. Byun, Y.; Whiteside, S., Ascorbyl palmitate-β-cyclodextrin inclusion complex as an oxygen scavenging microparticle. Carbohydrate Polymers 2012, 87 (3), 2114-2119.DOI: 10.1016/j.carbpol.2011.10.037
12. Ranjan, S.; Dasgupta, N.; Chakraborty, A.; Melvin Samuel, S.; Ramalingam, C.; Shanker, R.; Kumar, A., Nanoscience and nanotechnologies in food industries: opportunities and research trends. J Nanopart Res 2014, 16 (6), 1-23. DOI: 10.1007/s11051-014-2464-5
13. Kelly, K. L.; Coronado, E.; Zhao, L. L.; Schatz, G. C., The Optical Properties of Metal Nanoparticles: The Influence of Size, Shape, and Dielectric Environment. The Journal of Physical Chemistry B 2002, 107 (3), 668-677. DOI: 10.1021/jp026731y

