Following up on our recent Nanowerk Spotlight on nanofoods, new research shows that consumers could be exposed to nanoparticles in food by a much larger degree than has been expected so far.
For a modern consumer it is hard to avoid titanium dioxide (TiO2) – a widely used additive in food, personal care and other household products. Approximately 7 million tons of bulk TiO2 are produced annually and used as white pigment in order to provide whiteness and opacity to products such as paints, coatings, plastics, papers, inks, foods, pills, as well as most toothpastes.
In cosmetic and personal care products, it is used as a pigment, sunscreen and a thickener. TiO2 also is a photocatalyst; can oxidize oxygen or organic materials directly; and is superhydrophilic. You can therefore expect to see it increasingly used in paints, glass coatings, cement, tiles and ceramics, catalysts for air and water purification.
Due to increased performance (for instance better absorption and transparency of sun screens) and new uses (e.g. as nanocrystals in solar cells), the demand for TiO2 nanomaterials is growing. Roughly 50,000 tons of TiO2 nanoparticles were produced in 2010 and this amount is expected to grow to over 200,000 tons by 2015.
"Many applications of titanium dioxide would benefit from smaller primary particle sizes, and we can expect the percentage of TiO2 that is produced in or near the nano range to increase," Paul Westerhoff, a professor in the School of Sustainable Engineering and The Built Environment at Arizona State University and Senior Sustainability Scientist to the Global Institute of Sustainability, tells Nanowerk.
"TiO2 nanomaterials in foods, consumer products, and household products are discharged as feces/urine, washed off of surfaces, or disposed of to sewage that enters wastewater treatment plants. While these plants capture most of the TiO2, nanoparticles measuring between 4 and 30 nm were still found in the treated effluent. These nanomaterials are then released to surface waters, where they can interact with living organisms."
Westerhoff points out that, although the release of TiO2 nanomaterials to the environment has been shown qualitatively, quantification of how much is released is difficult.
In a recent paper in Environmental science & Technology ("Titanium Dioxide Nanoparticles in Food and Personal Care Products"), the scientists quantify the amount of titanium in common food products, derive estimates of human exposure to dietary (nano-) TiO2, and discuss the impact of the nanoscale fraction of TiO2 entering the environment.
Specifically, the team analyzed titanium dioxide in foods and from food suppliers using advanced instrumentation to assess what fraction of the materials was less than 100 nm in size (whether it was aggregated or individual nanoparticles).
"Of course, exposure to titanium dioxide depends largely on dietary habits, and in special cases the exposure could be several hundreds of milligrams per day," says Westerhoff.
The researchers' conclusion is that it appears that pigment TiO2 represents an enormous source of nanoscale TiO2 entering sewage systems, rivers, landfills, and other sensitive environmental compartments.
"It also appears that through surface modifications food-grade TiO2 (E171) is more readily dispersed into water than other TiO2 nanomaterials – such as P25, which has been used in many environmental and toxicity studies – which potentially influences TiO2 fate, transport, and toxicity," Westerhoff notes. "Therefore, more environmental ecotoxicology and fate studies should use the fraction of smaller sized TiO2 in pigments because exposure to these materials is likely to be much higher and more representative than exposure to P25."
The research team suggests that future work should investigate other nanoscale food and personal care additives and that the research community should work towards understanding their surface chemistry and behavior in the environment.
By Michael Berger
Fonte: NanoWerk
For a modern consumer it is hard to avoid titanium dioxide (TiO2) – a widely used additive in food, personal care and other household products. Approximately 7 million tons of bulk TiO2 are produced annually and used as white pigment in order to provide whiteness and opacity to products such as paints, coatings, plastics, papers, inks, foods, pills, as well as most toothpastes.
In cosmetic and personal care products, it is used as a pigment, sunscreen and a thickener. TiO2 also is a photocatalyst; can oxidize oxygen or organic materials directly; and is superhydrophilic. You can therefore expect to see it increasingly used in paints, glass coatings, cement, tiles and ceramics, catalysts for air and water purification.
Due to increased performance (for instance better absorption and transparency of sun screens) and new uses (e.g. as nanocrystals in solar cells), the demand for TiO2 nanomaterials is growing. Roughly 50,000 tons of TiO2 nanoparticles were produced in 2010 and this amount is expected to grow to over 200,000 tons by 2015.
"Many applications of titanium dioxide would benefit from smaller primary particle sizes, and we can expect the percentage of TiO2 that is produced in or near the nano range to increase," Paul Westerhoff, a professor in the School of Sustainable Engineering and The Built Environment at Arizona State University and Senior Sustainability Scientist to the Global Institute of Sustainability, tells Nanowerk.
"TiO2 nanomaterials in foods, consumer products, and household products are discharged as feces/urine, washed off of surfaces, or disposed of to sewage that enters wastewater treatment plants. While these plants capture most of the TiO2, nanoparticles measuring between 4 and 30 nm were still found in the treated effluent. These nanomaterials are then released to surface waters, where they can interact with living organisms."
Westerhoff points out that, although the release of TiO2 nanomaterials to the environment has been shown qualitatively, quantification of how much is released is difficult.
That is why he and his team, together with collaborators from ETH Zurich and NTNU Trondheim, have started to fill the existing knowledge gaps that exist regarding commonly used sources of TiO2 materials.
In a recent paper in Environmental science & Technology ("Titanium Dioxide Nanoparticles in Food and Personal Care Products"), the scientists quantify the amount of titanium in common food products, derive estimates of human exposure to dietary (nano-) TiO2, and discuss the impact of the nanoscale fraction of TiO2 entering the environment.
Specifically, the team analyzed titanium dioxide in foods and from food suppliers using advanced instrumentation to assess what fraction of the materials was less than 100 nm in size (whether it was aggregated or individual nanoparticles).
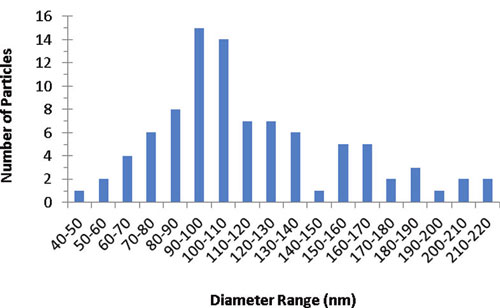 |
| Distribution of primary particle size of food grade titanium dioxide (E171). Analysis indicates that 36% of the particles were less than 100 nm in at least one dimension. (Reprinted with permission from American Chemical Society) |
For their experiments, the researchers selected a wide range of white foods from grocery stores in the U.S. Some of the foods were labeled as containing TiO2, and others were not but the primary product or surface coatings (e.g., icings) had a white color.
All 89 foods were digested using microwave methods (in a beaker with hydrogen peroxide and hydrofluoric acid), and their titanium concentration was determined.
They found that roughly 36% of food-grade TiO2 (E171) consists of particles which are less than 100 nm in at least one dimension and that it readily disperses in water as fairly stable colloids.
The scientists' simulated exposure to TiO2 for the U.S. population shows an average of 1-2 mg TiO2 per kilogram body weight per day for children under the age of 10 years and approximately 0.2-0.7 mg TiO2/kgbw/ day for the other consumer age groups.
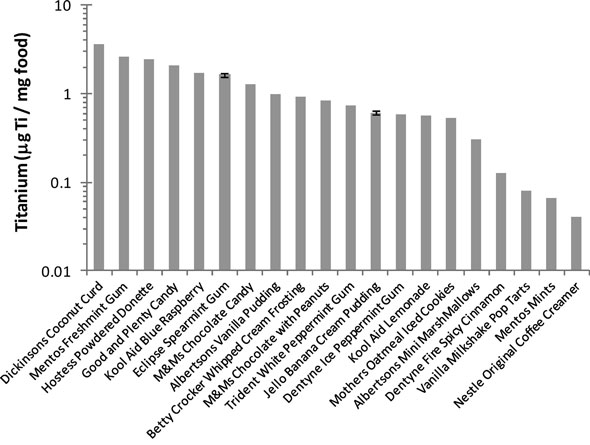 |
| Normalized titanium concentration in food products (top 20 products). (Reprinted with permission from American Chemical Society |
The scientists' simulated exposure to TiO2 for the U.S. population shows an average of 1-2 mg TiO2 per kilogram body weight per day for children under the age of 10 years and approximately 0.2-0.7 mg TiO2/kgbw/ day for the other consumer age groups.
"Of course, exposure to titanium dioxide depends largely on dietary habits, and in special cases the exposure could be several hundreds of milligrams per day," says Westerhoff.
"Because our measurements showed that roughly 36% of the particles in E171 may be in the nano range, we can presume a large exposure to nano-TiO2."
The researchers' conclusion is that it appears that pigment TiO2 represents an enormous source of nanoscale TiO2 entering sewage systems, rivers, landfills, and other sensitive environmental compartments.
"It also appears that through surface modifications food-grade TiO2 (E171) is more readily dispersed into water than other TiO2 nanomaterials – such as P25, which has been used in many environmental and toxicity studies – which potentially influences TiO2 fate, transport, and toxicity," Westerhoff notes. "Therefore, more environmental ecotoxicology and fate studies should use the fraction of smaller sized TiO2 in pigments because exposure to these materials is likely to be much higher and more representative than exposure to P25."
The research team suggests that future work should investigate other nanoscale food and personal care additives and that the research community should work towards understanding their surface chemistry and behavior in the environment.
By Michael Berger
Fonte: NanoWerk
