Noble metal nanoparticles such as gold, silver or platinum are widely used by scientists to develop novel applications in sensing, energy, spectroscopy, and catalysis. For instance, the combination of metal nanoparticles and carbon nanomaterials – graphene and nanotubes – has met with great interest in the area of biosensor applications (see for instance our Nanowerk Spotlight "Low cost graphite alternative to fabricating nanotechnology biosensors") as well as composite fabrication for light-energy conversion (see our Nanowerk Spotlight: "Photoactive graphene-semiconductor composites"). In these applications, researchers make use of the formation of organic/inorganic hybrid nanosystems by incorporating metal nanoparticles in or onto the graphitic structures of carbon nanotubes (CNTs) or graphene.
This work shows that graphene not only can serve as a catalytic support materials for the gold nanoparticles but also lower the temperature required for CNT synthesis.
Researchers have now discovered a novel phenomenon whereby graphene can be catalytically transformed into carbon nanotubes by gold nanoparticles at relatively low temperatures. Reporting their findings in a recent edition of ACS Nano ("Catalytic Conversion of Graphene into Carbon Nanotubes via Gold Nanoclusters at Low Temperatures"), a team led by Alexandru Biris, Sturgis Endowed Chair of Nanosciences at the University of Arkansas at Little Rock, reports that the 2D graphitic structure of the graphene can be transformed into 1D tubular shape at temperatures as low as 500°C.
"We have clearly demonstrated, for the first time to our best knowledge, that graphene structures play a major role in the catalytic activity of gold nanoparticles and that they can be responsible for the catalytic formation of carbon nanotubes," Biris tells Nanowerk.
Enkeleda Dervishi, Research Assistant Professor at the UALR Nanotechnology Center, the first and lead author of the paper, says that "based on our recent findings, we believe that graphene can be converted into tubular nanotube-like structures without the need of any additional carbon source, but only in the presence of the gold nanoparticles at relatively low catalytic temperatures of 500°C."
The authors point out that gold has been previously found to be a challenging catalytic element in the synthesis of CNTs and other carbon nanostructures.
"The facile method presented in our recent paper provides a methodology for the synthesis of CNTs/graphene composites – without including substrates such as SiO2 – at a low temperature and at atmospheric pressure, utilizing only a – noncarbon containing – carrier gas."
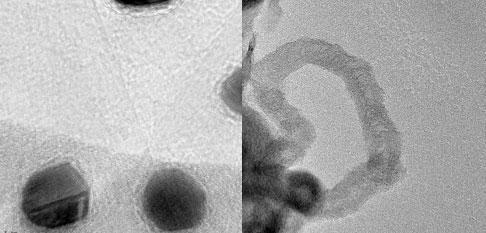 |
| Gold nanoparticles on the graphene layers (left) and carbon nanotubes produced during this catalytic process from graphene (right) (Images: UALR Nanotechnology Center) |
This work shows that graphene not only can serve as a catalytic support materials for the gold nanoparticles but also lower the temperature required for CNT synthesis.
"We are now focusing on further improving the actual catalytic growth process" says Biris.
When the team used SiO2 substrates coated with gold nanoparticles under the same reaction conditions they found to produce no CNTs or other tubular structures at any temperature.
They are now experimenting with other metal nanoparticles to see if they can also be used to modify the catalytic reaction during the synthesis of various carbon nanomaterials.
The synthesis of nanotubes at low temperatures could open significant technological directions.
"Such findings could be the foundation for new technologies used in a number of applications, ranging from capacitors, fuel cell membranes, and solar energy, to nanobiomedicine," say the authors. According to Biris, "these applications could be further improved by using this newly described multicomponent system formed of nanomaterials with 0, 1, and 2-dimensional morphologies."
The research was supported financially by NSF-EPSCOR, DOE and ASTA.
Fonte: NanoWerk
