Scientists at the Agricultural Research Service’s Cotton Chemistry and Utilization Research Unit (CCUR) in New Orleans, Louisiana, have a long history of research successes leading to advances in the use, manufacturing, and quality of cotton fiber.
 |
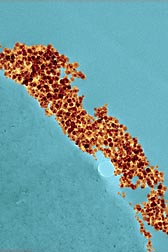
| Scanning electron microscope image of silver nanoparticles on the surface of cotton. The silver helps cotton fabric resist microbial growth. (D2468-1) |
For example, groundbreaking studies led by chemist Ruth Benerito at the Cotton Chemical Reactions Laboratory (CCUR’s predecessor), starting in the 1950s, gave rise to easy-care, permanent-press clothing and other consumer-friendly improvements that helped cotton better compete with synthetic fibers, like polyester and nylon.
New challenges and consumer demands have since emerged, but the ARS lab’s tradition of excellence and innovation in research continues.
Under the leadership of Brian Condon, CCUR researchers today are leveraging the latest developments in nanotechnology to bring cotton into the 21st century. Foreseeable applications range from the purely functional—like better shrink resistance—to the truly futuristic, such as fabrics made of cotton-optical fiber blends that can change color.
Flame-Retardant Coating
|
In one ongoing project, Condon and CCUR chemist SeChin Chang are collaborating with Texas A&M University (TAMU) scientists to evaluate a first-of-its-kind, environmentally friendly flame retardant for cotton apparel and durable goods.
Halogenated flame retardants have been among the most widely used chemical treatments for cotton. But there’s been a push to find alternatives that are not only more benign, but that also avoid imparting the same stiffness to fabric characteristic of some chemical treatments. For these and other reasons, “the textiles industry would like to move away from using halogenated flame retardants,” says Condon.
Made of water-soluble polymers, nanoscale clay particles, and other “green” ingredients, the ARS-TAMU flame retardant is applied as a nanocoating that reacts to open flame by rapidly forming a swollen, charred surface layer. This process, known as “intumescence,” stops the flame from reaching underlying or adjacent fibers.
A team led by Jaime Grunlan at TAMU’s Department of Mechanical Engineering, in College Station, Texas, originally developed the intumescent nanocoating using a layer-by-layer assembly. In this procedure, alternating layers of positively and negatively charged ingredients, including clay particles 50-100 nanometers wide, are deposited onto the surface of a desired material. The result is a striated nanocoating that, when viewed under a scanning electron or other high-powered microscope, resembles the stacked layers of a brick wall.
|
Condon’s interest was piqued after listening to Grunlan discuss his team’s research at a recent American Chemical Society meeting, and he approached the TAMU professor about potential benefits to cotton. That conversation, in turn, led to a cooperative research project enabling Condon and Chang to evaluate the nanocoating at CCUR.
Treating cotton for flame resistance isn’t a recent concept, adds Condon, whose lab is part of the ARS Southern Regional Research Center in New Orleans. In fact, some of the most successful early treatments were born of research conducted by Benerito and colleagues there several decades ago. (See “Cross-Linking Cotton,” Agricultural Research, February 2009, pp. 10-11.) Condon coauthored a 2011 ACS Nano paper on the potential of intumescent coatings together with Chang, Grunlan and his TAMU team, and Alexander Morgan of the University of Dayton Research Institute in Ohio.
Early trials of the nanocoating using standard flame-resistance tests are promising. In one case, 95 percent of treated cotton fabric remained intact after exposure to flame, whereas the untreated fabric used for comparison was completely destroyed
“What we’re investigating now is how well it will perform after repeated launderings of treated fabric,” says Condon. “After all, the coating contains clay, and that’s something detergents are made to remove.”
Even if the coating does eventually wash out and the treated fabric loses its flame resistance, the nanotech approach could still be used to protect textiles and durable goods that aren’t frequently washed, such as upholstery, mattress pads, box spring covers, automotive interiors, and firefighter coats.
|
Tapping Silver’s Antimicrobial Properties
On another nanotech front, Condon and CCUR engineer Sunghyun Nam are investigating a way to inhibit microbial growth in cotton using silver particles ranging in size from about 2 to 6 nanometers. Silver nanoparticles have been used as an antimicrobial agent in many products, including clothes, plastic food containers, and medical textiles. The methods of producing them, however, have mostly relied on the use of toxic agents and organic solvents.
Condon’s team has explored an alternative approach using an environmentally friendly agent, polyethylene glycol, and water as a solvent to generate silver nanoparticles of the desired size. Condon and Nam reported on this method in the Journal of Nanoparticle Researchtogether with engineer Dharnidhar Parikh, formerly with CCUR. Also under investigation is a new method by which the nanoparticles form directly on cotton fiber, eliminating handling and storage of the particles before application. “This is a leg up for cotton over the synthetics,” which have not been amenable to silver nanoparticle treatment, says Condon.
|
From Nanotech to Biotech
The CCUR scientists have also been busy on the biotech front.
In one project, CCUR chemist Vince Edwards, together with Condon, devised a treatment for impregnating nonwoven cotton fabrics with lysozyme, an enzyme that slices open the cell walls of microorganisms, killing them—including microbes that cause infection or odor. That same cell-slicing capacity may also be used for biodefense applications that deactivate nerve agents—essentially by chewing them up, or “hydrolyzing” them, he adds. By adding the lysozyme to cottons, the resulting nonwovens could have these bactericidal and detoxifying properties.
In another biotech project, Condon and CCUR chemist Michael Easson are experimenting with equipment that generates an ultrasonic field of mechanical energy (similar to that used to clean jewelry) to improve the biobased processing of raw, or “greige,” cotton using enzymes, like cellulase. Chemical processing agents are now used to strip away waxes, pectins, fats, and other fiber components that can hinder subsequent dying procedures and diminish the quality of finished cotton products. But the waste these “wet chemistry” methods generate is a concern.
“We’re interested in enzymes as an environmentally friendly alternative, and we found that subjecting a solution of the enzymes to a field of ultrasonic energy can speed up their reaction rates,” says Condon.
In experiments with lint fibers, for example, use of ultrasonic energy increased the bioprocessing efficiency of cellulase by 22 percent. The same concept can also work to improve the conversion of cotton sugars into biofuels—a potential value-added market for 2 million tons of U.S. cotton gin waste generated annually.
Besides publishing their findings in scientific journals, Condon’s team is actively seeking commercial partners to help usher these advances into the marketplace—all with an eye towards assuring the viability of America’s $25 billion cotton industry at a time of increasing production costs, dwindling resources, and global competition.—By Jan Suszkiw, Agricultural Research Service Information Staff.
This research is part of Quality and Utilization of Agricultural Products, an ARS national program (#306) described at www.nps.ars.usda.gov.
Brian Condon is in the USDA-ARS Cotton Chemistry Research Unit, Southern Regional Research Center, 1100 Robert E. Lee Blvd., New Orleans, LA 70124; (504) 286-4540.
"Cotton Gets Nanotech and Biotech Treatment in New Orleans" was published in the April 2012 issue of Agricultural Research magazine.